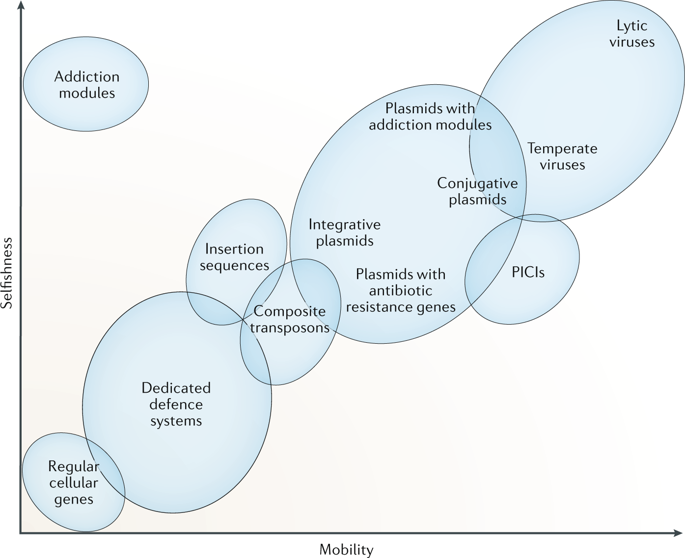
All cellular life forms are afflicted by diverse genetic parasites, including viruses and other types of mobile genetic elements (MGEs), and have evolved multiple, diverse defence systems that protect them from MGE assault via different mechanisms. Here, we provide our perspectives on how recent evidence points to tight evolutionary connections between MGEs and defence systems that reach far beyond the proverbial arms race. Defence systems incur a fitness cost for the hosts; therefore, at least in prokaryotes, horizontal mobility of defence systems, mediated primarily by MGEs, is essential for their persistence. Moreover, defence systems themselves possess certain features of selfish elements. Common components of MGEs, such as site-specific nucleases, are ‘guns for hire’ that can also function as parts of defence mechanisms and are often shuttled between MGEs and defence systems. Thus, evolutionary and molecular factors converge to mould the multifaceted, inextricable connection between MGEs and anti-MGE defence systems.
E. V. K. thanks W. Gofna (tel Aviv University) and E. Westra (University of Exeter) for inspiring discussions. E. V. K., K. S. M., and Y. I. W. are supported by the intramural research Program funds of the U.S. National institutes of health (U.S. Department of health and human services). M. K. was supported By the national agence Nationale de la Recherche (France) project ENVIRA (no. ANR-17-SE15-0005-01).
The manuscript was written by E. V. K. and M. K. All authors researched the data for the article and contributed to the discussion of the content and review/ edit of the manuscript before submitting.
The authors do not claim any competing interests.
Nature Reviews Genetics thanks K. Forsberg and other, anonymous, reviewer (s) for their contributions to the peer review of this work.
Springer Nature remains neutral on jurisdictional claims in published maps and institutional affiliations.
System toxin-antitoxin, which emoxipinum viral infection, causing the sleeping optimost or cell death.
The immune response to itself (that is, the components of the body itself instead of the components of mobile genetic elements). Autoimmune immunity stems from the failure of self-defense mechanisms and non-discrimination.
Prokaryotic adaptive immune systems that create an immune memory Bank by integrating foreign genome fragments into CRISPR arrays and use them to recognize and inactivate cognac foreign nucleic acid.
As for exaptation, it is a set of biological function for a function that is different from the function for which it was chosen.
(HGT). Gene transfer between organisms by any means other than vertical transmission from parents to offspring.
(ICE). A diverse class of mobile genetic elements present in numerous bacteria that are integrated into bacterial chromosomes and inherited vertically but retain the ability to excise and move horizontally by conjugation.
Viruses that, at the end of their breeding cycle, Lisa and kill the infected cell.
(IgE.) Genetic elements that are prone to change of location within a single genome (e.g. transposons) or to horizontal transmission between host cells (e.g. viruses and plasmids). Many IGE cover genes by mediating self-replication.
(RM systems). A variety of prokaryotic defense systems that contain various modification components (typically DNA methyltransferases) that modify and thus protect RNA, and restriction endonucleases that cleave to unmodified, foreign DNA.
Viruses that integrate into the host genome, forming proviruses, but retain the ability of induction with a subsequent cycle.
(TA modules). Genetic elements that consist of a toxin component (often a nuclease) and an antitoxin component that binds and transiently inactivates the toxin. When an antitoxin is inactivated, usually under stress, the toxin causes dormant cells or death.
Be the first to comment on "Evolutionary entanglement of mobile genetic elements and host defense systems: weapons for hire"